Consider the following unbalanced equation: 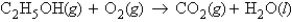 If 1.86 g of ethanol reacts with 14.3 g of oxygen, how many moles of water are produced?
If 1.86 g of ethanol reacts with 14.3 g of oxygen, how many moles of water are produced?
Definitions:
Plasmodesmata
Microscopic channels allowing transport and communication between plant cells, enabling the sharing of water, nutrients, and biochemical signals.
Intercellular Junction
Specialized structures that connect adjacent cells together, facilitating communication and the passage of molecules between cells.
Small Molecules
Organic compounds with low molecular weight, often used in pharmacology for their ability to modulate biological processes.
Q2: Balance the equation for the reaction of
Q5: Consider equal mole samples of dinitrogen monoxide,
Q26: You have 3.00 L of a 3.48
Q29: What is the expected ground-state electron configuration
Q30: What volume of 18.0 M sulfuric acid
Q32: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6421/.jpg" alt="For the
Q36: Express <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6421/.jpg" alt="Express in
Q37: You transfer a sample of gas at
Q160: Give the formula for chromium(III) iodide.
Q202: The name for Ba(NO<sub>3</sub>)<sub>2</sub> is<br>A) barium dinitrate<br>B)