Ibuprofen is a weak acid with a pKa of 4.9 (shown with the ionizable hydrogen with a star) . It is absorbed through the stomach and the small intestine as a function of polarity-charged and very polar molecules are absorbed slowly; neutral hydrophobic molecules absorb quickly. If the stomach pH is about 1.5 and the small intestine pH is about 6, where (and why) will more ibuprofen be absorbed into the bloodstream? 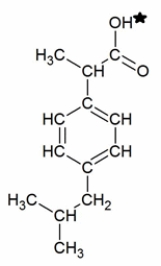
Definitions:
Q7: Which statement is NOT true about glutamine
Q10: Which statement does NOT describe a strategy
Q31: The processes of oxidative phosphorylation coupled with
Q41: Which statement is FALSE regarding ATP synthase?<br>A)
Q43: Which compound can be described as glucogenic?<br>A)
Q51: Which amino acid can be considered to
Q67: Which statement is FALSE regarding glutamine synthetase?<br>A)
Q88: In skeletal muscle, phosphocreatine functions as:<br>A) a
Q88: Which enzymatic activity would be decreased
Q99: Which intermediate of the citric acid cycle