The standard reduction potential for ubiquinone (Q or coenzyme Q) is 0.045 V, and the standard reduction potential (E'°) for FAD is -0.219 V. Using these values, show that the oxidation of FADH2 by ubiquinone theoretically liberates enough energy to drive the synthesis of ATP. The Faraday constant, 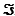 , is 96.48 kJ/V · mol. G'° for ATP synthesis is +30.5 kJ/mol.
, is 96.48 kJ/V · mol. G'° for ATP synthesis is +30.5 kJ/mol.
Definitions:
Legitimate Power
Power that is based on position and mutual agreement; agent and target agree that the agent has the right to influence the target.
Mutual Agreement
A mutual agreement involves a concord or pact between two or more parties, where each party has a clear understanding and is in accord about the terms and conditions discussed.
Agent's Position
The status or role of an individual acting on behalf of another person or an entity in a particular situation.
Referent Power
A form of influence that derives from the attractiveness and charisma of the leader, where followers identify with or admire the leader.
Q8: Briefly describe the "lipid burden" hypothesis that
Q23: Which statement is NOT true of the
Q30: Describe what happens at photosystem I from
Q45: Which amino acid is NOT considered
Q57: Which statement is TRUE of sphingolipid synthesis?<br>A)
Q62: Explain with an appropriate diagram why amphipathic
Q83: What is the CORRECT terminology for reactions
Q83: In photophosphorylation, absorption of light energy in
Q89: Briefly explain the role of phosphocreatine in
Q97: The steps of glycolysis between glyceraldehyde 3-phosphate