The standard reduction potential for ubiquinone (Q or coenzyme Q) is 0.045 V, and the standard reduction potential (E'°) for FAD is -0.219 V. Using these values, show that the oxidation of FADH2 by ubiquinone theoretically liberates enough energy to drive the synthesis of ATP. The Faraday constant, 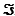 , is 96.48 kJ/V · mol. G'° for ATP synthesis is +30.5 kJ/mol.
, is 96.48 kJ/V · mol. G'° for ATP synthesis is +30.5 kJ/mol.
Definitions:
Shortcut Menu
A context-sensitive menu that appears upon right-clicking, providing quick access to frequently used commands or options.
Insert Field
An action or command in software that allows the user to add a new data field into a document or database.
Insert Column
A function in spreadsheet software that allows users to add a new column to their data table.
Add Column
The process of inserting a new column into a table or spreadsheet, expanding its structure.
Q7: What enzyme converts arginine into urea and
Q34: Which compound links glycolysis and nucleotide synthesis?<br>A)
Q37: Which protein associated with electron transport below
Q42: Why is an anaerobic fate of pyruvate
Q50: Thyroxine is a competitive inhibitor of malate
Q61: The synthesis of methionine originates from
Q62: A possible product of isoleucine catabolism
Q70: Draw the structure of 5'-UMP (uridylic acid).
Q84: Describe the role of fructose 2,6-bisphosphate in
Q88: A cell that is unable to synthesize