Considering the limiting reactant concept,how many moles of sodium chloride are produced from the reaction of 0.750 mol of sodium and 0.500 mol of chlorine gas? 2 Na(s) + Cl₂(g) 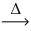 NaCl(s)
NaCl(s)
Definitions:
Municipal Bylaw
A law passed by a local government authority that applies to residents within its jurisdiction.
Liquor Licence
An official permit required to sell alcoholic beverages within certain regulations.
Mediation
A form of alternative dispute resolution where a neutral third party helps disputing parties reach a mutually agreeable solution.
Procedural Fairness
The requirement for legal and administrative decisions to be made following processes that are fair, proper, and established, ensuring individuals are treated justly.
Q12: How many hydrogen molecules are in 2.75
Q50: What are the predicted products from the
Q58: Part of one's self-concept that is based
Q76: Express the exponential number 5.15 x 105
Q81: What is the predicted ionic charge of
Q129: Which of the following metals does not
Q133: Which of the following is a situation
Q138: Which of the following steps is necessary
Q147: What is the volume of oxygen gas
Q173: Which of the following is NOT one