Considering the limiting reactant concept,how many moles of cobalt(III) oxide are produced from the reaction of 1.00 mol of cobalt and 1.00 mol of oxygen gas? 4 Co(s) + 3 O₂(g) 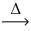 2 CO₂O₃(s)
2 CO₂O₃(s)
Definitions:
War Debts
The money owed by several countries as a result of borrowing to finance their involvement in World War I and other conflicts.
Reparations Issue
The debate and discussion regarding compensation for groups that have suffered injustices and harms, such as slavery or colonization, focusing on the acknowledgment of past wrongs and the provision of redress.
First World War
A global conflict that took place from 1914 to 1918, primarily involving European nations, and is known for introducing widespread military conscription and trench warfare.
American Labor Force
The total number of workers, including both the employed and the unemployed, within the United States.
Q6: What are the predicted prodcts from the
Q18: Which of the following quantities can be
Q20: Aqueous H₂Se is classified as which of
Q42: What volume of oxygen gas reacts with
Q53: How many molar masses of water are
Q71: Dr.Braun has been treating a child with
Q74: If 0.300 mol of lead combines with
Q103: Which of the following instruments has the
Q200: A research hypothesis proposes that consuming low-carbohydrate
Q207: Meta-analysis combines and analyzes data from many