Multiple Choice
Considering the limiting reactant,what is the mass of manganese produced from 25.0 g of manganese(IV) oxide (86.94 g/mol) and 25.0 g of aluminum metal? 3 MnO₂(l) + 4 Al(l) 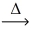 3 Mn(l) + 2 Al₂O₃(s)
3 Mn(l) + 2 Al₂O₃(s)
Definitions:
Related Questions
Q27: How many atoms of cobalt equal a
Q31: What is the ionic charge for the
Q32: What is the mass of insoluble lead(II)iodide
Q34: What is the systematic name for Br3O8?<br>A)bromine
Q46: The just-world hypothesis leads to a dispositional
Q50: Considering the limiting reactant concept,how many moles
Q122: What is the chemical formula for the
Q122: What is the mass in grams of
Q142: What term refers to the volume occupied
Q164: What is the term for a compound