Considering the limiting reactant,what is the volume of NO gas produced from 60.0 L of ammonia gas and 50.0 L of oxygen gas? (Assume constant conditions. ) 4 NH₃(g) + 5 O₂(g) 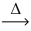 4 NO(g) + 6 H₂O(g)
4 NO(g) + 6 H₂O(g)
Definitions:
Entrepreneurial Spirit
An attitude and approach characterized by innovation, risk-taking, and the ability to see and seize opportunities for business or personal gain.
Entrepreneur
An individual who undertakes the creation, organization, and ownership of an innovative business with potential for growth and financial reward.
Franchising
A business model that allows individuals to operate their own outlets of a larger chain, sharing in the brand, products, and operational support.
Royalties
Payments made to the original creators or owners of intellectual property for the right to use their work.
Q13: How many moles of carbon monoxide react
Q23: What is the chemical formula for the
Q29: How many moles of oxygen gas react
Q32: Which of the following instruments provides an
Q36: The term _ is unregulated and so
Q58: What does it mean to say that
Q59: Which of the following is evidence for
Q73: How many molecules of bromine liquid,Br₂,have a
Q84: What is the coefficient of chlorine gas
Q113: Graphology,or handwriting analysis,uses precise measurements in order