How many moles of water are produced from 0.100 mol pentane,C5H₁₂? __C5H₁₂ (g) + __O₂(g) 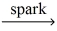 __CO₂(g) + __H₂O(g)
__CO₂(g) + __H₂O(g)
Definitions:
Competitive Firms
Companies that operate in markets where no single firm has the power to influence the price of goods and services significantly.
Monopolistic Firms
Companies that have significant control over the market for a particular good or service, allowing them to influence price and production levels.
Economic Inefficiency
A situation where resources are not used in the most productive way, often leading to waste or a loss of potential output.
Minimum Average Total Cost
The lowest point on the average total cost curve, where a firm is most efficiently combining resources to produce goods or services.
Q3: What volume of oxygen gas reacts to
Q18: Behavior that occurs when we change our
Q52: The phosphite ion,PO₃3-,is classified as which of
Q66: The formula for mustard gas used in
Q90: How are twin studies used to estimate
Q145: Which of the following metals reacts with
Q159: What type of chemical reaction is illustrated
Q170: Because the people who are willing to
Q186: Mark has a strong desire to quit
Q192: Experiments showing the effects of group pressure