What volume of propane gas,C₃H₈,reacts to give 5.00 L of carbon dioxide gas? (Assume temperature and pressure remain constant. ) __C₃H₈(g) + __O₂(g) 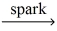 __CO₂(g) + __H₂O(g)
__CO₂(g) + __H₂O(g)
Definitions:
Quick (Acid Test) Ratio
A liquidity measure evaluating a company's ability to cover its short-term liabilities with its most liquid assets.
Current Liabilities
Current liabilities are a company's debts or obligations that are due to be paid to creditors within one year.
Temporary Investments
Securities or assets that a company intends to sell within a short period, typically one year, to generate income.
Net Receivables
The amount of money expected to be received from all outstanding accounts receivable after deducting allowances for doubtful accounts.
Q13: What is the term for the smallest
Q21: What are the predicted products from the
Q33: If 1.775 g of arsenic react with
Q59: What is the number of manganese atoms
Q66: In the 1970s,a 13-year-old girl was rescued
Q99: If 1.823 g of cobalt metal reacts
Q123: The Hg2+ ion is classified as which
Q130: Considering the limiting reactant concept,how many moles
Q133: Which of the following is a situation
Q144: What is the chemical formula for the