Considering the limiting reactant concept,how many moles of copper(I) sulfide are produced from the reaction of 0.500 mol of copper and 0.750 mol of sulfur? 2 Cu(s) + S(s) 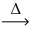 Cu2S(s)
Cu2S(s)
Definitions:
Permanent/Temporary
This refers to the nature of employment or status of objects wherein 'permanent' indicates indefinite duration and 'temporary' indicates a fixed duration.
Normal Balance
Normal Balance is the side (debit or credit) where increases to the account are recorded, depending on the account type.
Permanent/Temporary
Describes accounts in financial reporting; permanent accounts show ongoing financial status, while temporary accounts track revenues, expenses, and dividends over a specific period.
Normal Balance
The side (debit or credit) of an account that increases its balance, reflecting the account's role in the accounting equation.
Q31: Scholars of the past who wanted to
Q42: If 1.00 L of an unknown gas
Q60: A correlation coefficient of -1.73 means that<br>A)the
Q66: The formula for mustard gas used in
Q97: Julie noticed that the number of hours
Q106: Why is "tolerating uncertainty" considered an important
Q124: If the density of an unknown gas
Q137: What is the chemical formula for cobalt(II)bromide?<br>A)CO₂Br<br>B)CoBr₂<br>C)CO₃Br₂<br>D)CO₂Br3<br>E)none
Q187: Dr.Littman-Smith is conducting research in Kenya in
Q205: Which modern psychological perspective had its origin