Considering the limiting reactant,what is the mass of zinc sulfide (97.46 g/mol) produced from 0.750 g of zinc and 0.750 g of sulfur? Zn(s) + S(s) 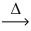 ZnS(s)
ZnS(s)
Definitions:
Elementary Units
The simplest, indivisible entities which a data set or a statistical analysis is concerned with.
Finite Result
An outcome or solution that is fixed and definitive, with limitations in terms of size, number, or extent.
Discrete Variable
A variable that can take on a countable number of distinct values and cannot take on every value within its range.
Discrete Random Variable
A variable that can take on a countable number of distinct values, often associated with experiments that have a finite set of possible outcomes.
Q3: What is the term for a method
Q23: Considering the limiting reactant,what is the volume
Q24: How many moles of oxygen react with
Q37: Add 7.77 g to 11.666 g and
Q53: Convert 155 x 105 to scientific notation.<br>A)1.55
Q133: What type of chemical reaction is illustrated
Q140: In an experiment,0.327 g of zinc metal
Q153: The compound NH₃ is classified as which
Q154: Terms used in hypotheses are given operational
Q169: What is the chemical formula for the