Considering the limiting reactant,what is the mass of zinc sulfide (97.46 g/mol) produced from 0.750 g of zinc and 0.350 g of sulfur? Zn(s) + S(s) 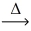 ZnS(s)
ZnS(s)
Definitions:
High-Context
A communication style in which most of the information is already in the person, with very little in the explicit, transmitted part of the message.
Low-Context
Communication style in which the majority of the information is conveyed through explicit verbal messages rather than contextual cues.
High-Content
Information or media that are rich in substance and significance, often providing comprehensive and deep insights on a subject.
Chain of Command
The hierarchy of authority in an organization that determines how instructions are passed down and accountability is enforced.
Q1: What type of chemical reaction is illustrated
Q43: The likelihood of lying about a touchy
Q45: Predict the chemical formula for radium selenide,given
Q49: Subtract 9.0 x 103 from 2.0 x
Q56: Which of the following is not a
Q65: What is the term for the substance
Q99: What is the term for a system
Q106: Which of the following elements occurs naturally
Q162: Alexia and Holly both plan to become
Q207: Meta-analysis combines and analyzes data from many