Considering the limiting reactant,what mass of cobalt(III) sulfide (214.07 g/mol) is produced from 0.750 g of cobalt and 0.350 g of sulfur? 2 Co(s) + 3 S(s) 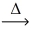 CO₂S3(s)
CO₂S3(s)
Definitions:
Commercial Extinction
A condition in which a species exists in such low numbers that it is no longer profitable to harvest.
Coral Reefs
Diverse underwater ecosystems held together by calcium carbonate structures secreted by corals, providing habitat and shelter for many marine species.
Biodiversity Of Oceans
The variety of life in the world's oceans and seas, including the ecosystems, species, and genetic diversity found within marine environments.
Habitat Degradation
The process by which a natural habitat becomes incapable of supporting its native species due to environmental pressures or human activities.
Q9: What is the volume of carbon dioxide
Q35: What is the term for the amount
Q44: Butyric acid is the odor of rancid
Q62: Multiply 2.505 cm by 1.75 cm by
Q85: What is the term for eliminating digits
Q90: What is the volume of 4.80 x
Q95: "Can I recall events from my childhood
Q96: How many significant digits are in the
Q133: What type of chemical reaction is illustrated
Q136: Critical thinking may be defined as<br>A)negative thinking