Multiple Choice
Considering the limiting reactant,what is the mass of manganese produced from 25.0 g of manganese(IV) oxide (86.94 g/mol) and 25.0 g of aluminum metal? 3 MnO₂(l) + 4 Al(l) 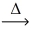 3 Mn(l) + 2 Al₂O₃(s)
3 Mn(l) + 2 Al₂O₃(s)
Understand the relationship between level of confidence and precision of the confidence interval.
Evaluate the influence of confidence intervals on hypothesis testing.
Assess the relationship between confidence level choices and their implications on research interpretation.
Identify misconceptions and limitations associated with hypothesis testing.
Definitions:
Related Questions
Q15: Which of the following is true about
Q90: Which of the following is not in
Q102: Stereotypes can be useful tools in the
Q103: How many moles of hydrogen gas react
Q126: Classify the following type of stoichiometry problem:
Q151: What is the chemical formula for strontium
Q162: Which of the statements below best describes
Q167: Deindividuation occurs when members of a group
Q202: A sample's representativeness is less critical than
Q207: Meta-analysis combines and analyzes data from many