Considering the limiting reactant,what is the volume of NO gas produced from 30.0 L of nitrogen gas and 20.0 L of oxygen gas? (Assume constant conditions. ) N₂(g) + O₂(g) 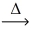 2 NO(g)
2 NO(g)
Definitions:
Demurrer
A motion for dismissal of a case on the grounds that a plaintiff has failed to state a claim for which relief can be granted.
Affirmative Defense
A set of circumstances that indicates that a defendant should not be held liable, even if the plaintiff proves all of the facts in a complaint.
Assumption of the Risk
A legal doctrine where an individual knowingly and voluntarily assumes the risk of an activity, thereby reducing the liability of others.
Demurrer
A legal objection that an opponent's point is irrelevant or invalid, while conceding the factual basis of the point.
Q33: If 1.775 g of arsenic react with
Q51: Which of the following represents 1 mol
Q57: Jill believes that Honda owners are thrifty
Q58: What is the systematic name for aqueous
Q100: Professor Turner wants to know if physiological
Q112: What is the systematic name for CdI₂?<br>A)cadmium
Q134: What is the chemical formula for tetraphosphorus
Q135: The S₂- ion is classified as which
Q148: What is the general term for any
Q186: One way in which organizations actively promote