Considering the limiting reactant,what is the volume of NO₂ gas produced from 3.00 L of NO gas and 2.00 L of oxygen gas? (Assume constant conditions. ) 2 NO(g) + O₂(g) 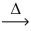 2 NO₂(g)
2 NO₂(g)
Definitions:
Nondiscriminating Monopolist
Refers to a monopolist who charges all consumers the same price for its product or service, as opposed to price discrimination practices.
Pure Monopolist
A market structure where a single seller controls the entire supply of a product or service, and no close substitutes exist.
Maximum Profits
The highest level of profit a firm can achieve when it operates at its most efficient production level and pricing strategy.
Price Discriminate
The practice of selling the same product or service at different prices to different customers, not based on differences in production costs.
Q20: Which of the following terms is a
Q34: What is the coefficient of hydrogen gas
Q75: What are the products from the following
Q77: An attitude is a belief about people,groups,ideas,or
Q86: Round off the following measurement to three
Q90: What are the products from the complete
Q93: In general,how many unit factors are required
Q110: How many moles of nitrogen dioxide gas
Q111: What is the Stock system name for
Q141: The decomposition of 1.500 g of baking