Considering the limiting reactant,what is the volume of NO gas produced from 40.0 L of ammonia gas and 40.0 L of oxygen gas? (Assume constant conditions. ) 4 NH₃(g) + 5 O₂(g) 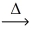 4 NO(g) + 6 H₂O(g)
4 NO(g) + 6 H₂O(g)
Definitions:
Scanner
A device that captures images from photographic prints, posters, magazine pages, and similar sources for computer editing and display.
Electrical Power
The generation, distribution, and use of electric energy.
Dialog Box
A small window that appears in a graphical user interface to communicate information to the user and receive input.
File Name
A label assigned to a digital document or file to distinguish it from others and to indicate its contents.
Q2: How many moles of calcium metal react
Q13: If 6.00 mol of cobalt combines with
Q15: Divide 11.375 g by 10.0 mL and
Q48: What are the products from the following
Q49: Subtract 9.0 x 103 from 2.0 x
Q70: What is the term for a substance
Q75: What is the chemical formula for tin(II)chlorate?<br>A)Sn(ClO₂)2<br>B)Sn(ClO₂)4<br>C)Sn(ClO₃)2<br>D)Sn(ClO₃)4<br>E)none
Q106: In an experiment,5.585 g of iron metal
Q113: In an experiment,1.201 g of charcoal reacts
Q130: Two separate studies investigated the relationship between