Considering the limiting reactant,what is the volume of NO gas produced from 50.0 L of ammonia gas and 60.0 L of oxygen gas? (Assume constant conditions. ) 4 NH₃(g) + 5 O₂(g) 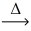 4 NO(g) + 6 H₂O(g)
4 NO(g) + 6 H₂O(g)
Definitions:
Sugar Reabsorption
The process by which the kidneys filter and reabsorb glucose back into the bloodstream, preventing excess sugar from being excreted in urine.
Threshold
A minimum amount of supplies to be maintained; also known as par level.
Afferent Arteriole
A small artery that carries blood toward the capillaries of the glomerulus in the kidney, playing a key role in regulating blood pressure and filtration rate.
Dialysis Solution
A fluid used in the process of dialysis, which is aimed at removing waste products and excess substances from the blood when the kidneys are not able to function efficiently.
Q32: One common disadvantage for experimental studies is
Q43: If 1.888 g of bismuth metal reacts
Q48: Distinguish between implicit and explicit attitudes.
Q79: Which common acid is sold in the
Q95: What is the volume of 2.75 x
Q108: Which of the following techniques can be
Q111: What is the coefficient of hydrogen gas
Q115: How is the just-world hypothesis related to
Q123: What term refers to the mass of
Q198: The group of mental health professionals who