What are the predicted products from the following decomposition reaction? Zn(ClO₃) 2(s) 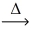
Definitions:
Alpha Decay
A type of radioactive decay in which an atomic nucleus emits an alpha particle (two protons and two neutrons bound together) and transforms into a different element.
Atomic Nucleus
The atomic nucleus is the small, dense region at the center of an atom, composed of protons and neutrons, and is the core where most of an atom's mass is concentrated.
Ionizing Power
The ability of a chemical element or electromagnetic radiation to ionize atoms or molecules by altering their charge through the removal or addition of electrons.
Beta Particles
High-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei during radioactive decay.
Q19: Which of the following elements has the
Q21: What are the predicted products from the
Q33: What is the volume of carbon dioxide
Q46: The formula for mustard gas used in
Q71: In general,how many unit factors are required
Q89: What is the term for ten followed
Q94: What does the acronym IUPAC represent?<br>A)Intermediate Unresolved
Q95: Which of the following is a transition
Q151: Which of the following is the electron
Q183: The Cl- ion is classified as which