Which of the statements listed below is true regarding the following redox reaction occurring in a nonspontaneous electrochemical cell? Br₂(l) + 2 NaCl(aq) 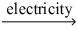 Cl₂(g) + 2 NaBr(aq)
Cl₂(g) + 2 NaBr(aq)
Definitions:
Risk Averse
a characteristic describing individuals or entities that prefer to avoid risks, often seeking safer or more predictable outcomes.
Women
Female human beings; also refers to gender-based social constructs and roles.
Experienced Managers
Individuals with significant expertise and knowledge in managing operations, teams, and making strategic decisions.
Logical Reasoning
The process of using a rational, systematic series of steps based on sound mathematical procedures and given statements to arrive at a conclusion.
Q23: If a drain cleaner has a pH
Q31: Which of the following is a general
Q73: What acid and base are neutralized to
Q77: What is the term for the value
Q88: Nitric acid is a strong acid,ammonium hydroxide
Q89: Which of the changes listed below will
Q97: Bombarding a target nucleus with a deuterium
Q102: Which of the changes listed has no
Q105: What is the term for that portion
Q174: What is the name of the following