Which of the statements listed below is true regarding the following redox reaction occurring in a nonspontaneous electrochemical cell? 3 C(s) + 2 CO₂O₃(l) 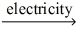 4 Co(l) + 3 CO₂(g)
4 Co(l) + 3 CO₂(g)
Definitions:
Accounts Receivable
Unpaid amounts from customers for goods or services that a business has already provided.
Net Income
The total earnings of a company after subtracting all expenses, taxes, and costs from total revenue.
Depreciation Expense
The allocated amount of the cost of an asset, spread out over the time it is expected to be used, reflecting its consumption, wear and tear, or obsolescence.
Indirect Method
In cash flow calculation, this method adjusts net income for non-cash transactions and changes in working capital to arrive at net cash provided by operating activities.
Q8: A sample of sodium carbonate is treated
Q15: The initial activity of a cobalt-60 sample
Q24: Which of the following illustrates the like
Q39: Which of the following is a potential
Q59: Which of the following molecular formulas corresponds
Q68: Given the reaction for manufacturing hydrogen peroxide,H₂O₂,which
Q81: If a light bulb in a conductivity
Q97: What functional group is present in the
Q121: Steelmaking plants and electric power plants that
Q123: What equilibrium constant applies to a reversible