Which of the statements listed below is true regarding the following redox reaction occurring in a nonspontaneous electrochemical cell? 3 C(s) + 4 AlCl3(l) 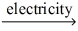 4 Al(l) + 3 CCl4(g)
4 Al(l) + 3 CCl4(g)
Definitions:
Resistance to Change
The act of opposing or struggling with modifications or transformations within an organization or in one's personal life.
Innovation Inertia
The resistance to change or the slowness in adopting new ideas and innovations within an organization.
Departmentalization
A process of grouping people with related job duties, skills, and experiences into different areas within the overall organizational structure.
Span of Control
A management concept that refers to the number of subordinates a supervisor or manager can effectively manage and control.
Q1: If the unknown quantity is g/mL,what is
Q9: What is the term for a solution
Q14: What is the [H+] in a blood
Q24: What is the equilibrium constant expression,Ki,for the
Q47: What radionuclide decays to Br-73 by positron
Q52: What is the mass of a 10.0%
Q65: Which of the following units is an
Q72: Which of the following illustrates the reaction
Q75: In 1967 a team of Russian scientists
Q91: Which of the following organic compounds is