Which of the statements listed below is true regarding the following redox reaction occurring in a nonspontaneous electrochemical cell? Br₂(l) + 2 NaCl(aq) 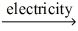 Cl₂(g) + 2 NaBr(aq)
Cl₂(g) + 2 NaBr(aq)
Definitions:
Functional Exchange
A process in which functions or services are exchanged between or among entities, often in the context of business or software systems, to improve efficiency or performance.
Software Rights
Legal rights related to the licensing, use, and distribution of software.
Forward Auctions
A type of auction where buyers bid against each other to procure a good or service, with the price typically increasing with each bid.
Prices
The amount of money required to purchase goods or services, determined by factors such as supply, demand, and production cost.
Q15: The initial activity of a cobalt-60 sample
Q25: What is the general equilibrium constant expression,Keq,for
Q40: Which of the following is a health
Q46: Which of the following is a strong
Q58: What is the term for the component
Q59: What is the molarity of a glucose
Q91: In the following reaction,which reactant is a
Q105: What is the term for a gas
Q148: What is the IUPAC name for CH₃-CH₂-CH₂-CH₂-C≡C-CH₂-CH₃?<br>A)octyne<br>B)3-octyne<br>C)4-octyne<br>D)5-octyne<br>E)none
Q178: What is the systematic name for CH₃-CCl₂-CH₃?<br>A)1,1-dichloropropane<br>B)1,2-dichloropropane<br>C)1,3-dichloropropane<br>D)2,2-dichloropropane<br>E)3,3-dichloropropane