Which of the statements listed below is true regarding the following redox reaction occurring in a nonspontaneous electrochemical cell? Br₂(l) + 2 NaCl(aq) 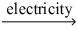 Cl₂(g) + 2 NaBr(aq)
Cl₂(g) + 2 NaBr(aq)
Definitions:
Informal Fallacy
A form of flawed logic where an argument feels emotionally or psychologically compelling yet fails to hold up under logical scrutiny.
Begs the Question
A logical fallacy where the argument's premise assumes the truth of the conclusion, instead of supporting it.
Popular Appeal
An appeal to popular opinion to gain support for our conclusion.
Famous Person
An individual who has gained widespread recognition and public attention.
Q17: What is the general equilibrium constant expression,Keq,for
Q42: In 1994 a team of German physicists
Q44: Apply the like dissolves like rule to
Q83: What is the symbol for the molar
Q95: What is the [OH-] in an ammonia
Q113: What is the pH of an aqueous
Q119: What is the estimated top speed of
Q131: What is the term for nuclear radiation
Q149: What is the product from the reaction
Q182: What is the term for a family