If 10.0 L of nitrogen gas at STP reacts completely with excess hydrogen gas,what is the volume of ammonia produced at 500.0 K and 500.0 atm? N₂(g) + H₂(g) 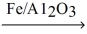 NH₃(g)
NH₃(g)
Definitions:
Young Adult
An individual in the life stage following adolescence, typically ranging from late teens to early thirties, characterized by physical maturity and the pursuit of independence.
Mole
A unit in chemistry used to express amounts of a chemical substance, based on the number of atoms or molecules.
Molecular Weight
A measure of the mass of a molecule, calculated as the sum of the atomic masses of all atoms constituting the molecule.
Grams
A metric unit of mass equal to one thousandth of a kilogram.
Q7: After balancing the following redox reaction,what is
Q10: Apply the like dissolves like rule to
Q11: Which substance listed below is the strongest
Q24: What is the term for the geometric
Q24: Which of the following illustrates the like
Q35: Which of the following expresses the molar
Q47: Which of the following is true of
Q70: What volume of 16 M acid must
Q112: Which of the following occurs naturally as
Q158: What is the total number of valence