Consider the following energy diagram for a reaction. 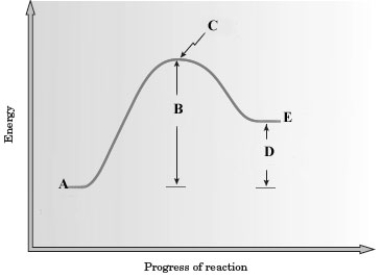
-Which of the following correctly represents this reaction?
Definitions:
Quantity Demanded
The total amount of a good or service that consumers are willing to purchase at a given price level in a given period.
Coefficient
A numerical value that multiplies a variable in an equation, serving as a measure of some property or characteristic.
Price Elasticity
Refers to how sensitive the quantity demanded of a good is to a change in its price.
Demand Curve
A graph showing the relationship between the price of a good or service and the quantity demanded for a given period.
Q8: Which of the following classes of compounds
Q15: Which of the following occurs when a
Q37: What is the formula of the compound
Q42: Which of the following will occur if
Q65: When a certain clear red-brown liquid passes
Q79: Consider a substance as represented by the
Q93: Assuming that the volumes are additive, what
Q95: Which of the following forms of radiation
Q107: Which of the following forms of radiation
Q129: In a particular titration experiment a 25.0