The following chemical reaction has reached equilibrium. 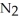
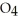 (g)
(g) 
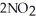 (g) Calculate the equilibrium constant, Keq, if the concentrations of
(g) Calculate the equilibrium constant, Keq, if the concentrations of 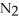
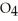 and
and 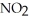 are 0.0238 M and 0.196 M, respectively.
are 0.0238 M and 0.196 M, respectively.
Definitions:
Ultimate Consumer
The final buyer who purchases products or services for personal use, not for resale.
Organizational Buyer
Refers to entities such as companies, governments, and institutions that purchase goods and services for use in the production of other products and services or for resale.
Marketing Concept
A philosophy that firms should analyze the needs of their customers and then make decisions to satisfy those needs, better than the competition.
Marketing Program
An organized, comprehensive plan that outlines the advertising, sales, and marketing efforts for a specific period of time, with the goal of achieving identified business objectives.
Q7: H₂PO₄- is the conjugate acid of PO₄2-.
Q9: 5.0 g LiI in 250 g of
Q12: How many grams of AgNO<sub>3</sub> are in
Q18: If a chemical reaction at an equilibrium
Q59: If the pressure of a given gas
Q65: Which of the following statements best describes
Q73: Joule<br>A)time<br>B)energy<br>C)electrical charge<br>D)mass<br>E)length<br>F)volume<br>G)temperature<br>H)density
Q76: Which of the following does not carry
Q89: 30.19 inches of Hg is equal to
Q92: A reaction is close to equilibrium when