Write an expression for the equilibrium constant (Keq) for the following reaction. 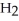 (g) +
(g) + 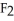 (g)
(g)  2HF (g)
2HF (g)
Definitions:
Q23: An unknown gaseous substance has a density
Q66: 855 torr is equal to _ cm
Q73: Rate = k[A]3/[B]<br>A)3<br>B)1<br>C)0<br>D)2<br>E)4
Q81: 1,4-diaminobenzene is one component used to make
Q94: Which of the following hydrocarbon molecules is
Q101: ethene<br>A)CH?CH?CH=CHCH?CH?<br>B)CH?=CHCH?CH?CH?CH?<br>C)CH?CH=CHCH?<br>D)CH?CH=CHCH?CH?<br>E)CH?=CHCH?<br>F)CH?CH=CHCH?CH?CH?<br>G)CH?=CHCH?CH?CH?<br>H)CH?=CHCH?CH?<br>I)CH?=CH?
Q105: 1-hexyne<br>A)CH?CH?C?CCH?CH?<br>B)HC?CCH?CH?CH?<br>C)CH?C?CCH?<br>D)CH?C?CH<br>E)HC?CCH?CH?CH?CH?<br>F)CH?C?CCH?CH?<br>G)CH?C?CCH?CH?CH?<br>H)HC?CH<br>I)HC?CCH?CH?
Q111: "Buffered" solutions are resistant to pH changes,
Q116: 1n + _ → 140Ba + 90Kr
Q118: 21.7 L occupied by _.<br>A)40.0 g of