For the following exothermic reaction:
CO + 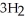

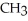 OH
OH
predict the direction of the reaction if:
a) temperature is decreased with the concentrations remaining the same.
b) a catalyst is added with the concentrations AND temperature remaining the same.
c) the concentration of 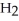 is halved.
is halved.
Definitions:
Private-Sector Differential
The differences in wages, working conditions, and employment benefits between jobs in the private sector versus those in the public sector.
Globalization
The process of increased interconnectedness and interdependence among countries, mainly through trade, investment, and cultural exchange.
Two-Tiered Wage
A pay scale system that offers a higher wage tier for current employees and a lower wage tier for new hires for the same job.
Monopoly Power
The ability of a single seller to influence the price and production levels in the market.
Q3: 0.123 g of a gas occupy a
Q14: Construct the complementary strand of this piece
Q25: In each of the following diagrams, the
Q35: Which of the following compounds can behave
Q40: The base in the forward reaction is
Q43: Which of the following is true about
Q54: Adding a catalyst increases the rate of
Q55: Uranium-238 can capture a neutron inside a
Q129: Which of the following is the most
Q136: Which of the following compounds is an