The following compound shows signals at 190.7, 140.9, 134.8, 130.9 and 129.5 in its broadband decoupled C-13 spectrum. Assign the signals to appropriate carbons. 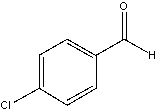
Definitions:
Molecular Polarity
Molecular polarity refers to the distribution of electrical charges over the atoms in a molecule, affecting its interaction with other molecules and solvents.
Solvent Interaction
The interaction between a solvent and solute which leads to the dissolution of the solute into the solvent.
Melting Point
The melting point is the temperature at which a solid substance transitions to a liquid state, indicative of the heat required to break the molecular bonds within the solid.
Electronegativity
A measure of an atom's ability to attract and hold onto electrons when it is part of a compound.
Q6: Provide the reagents necessary to carry out
Q10: Provide the structure for N-methylcyclohexanecarboxamide.
Q16: What is the IUPAC name for the
Q16: Predict the product for the following reaction.
Q29: Ethanoic acid is commonly known as _.
Q31: Predict the product for the following Claisen
Q45: Which is the most energetically favorable
Q66: Predict the major product for the following
Q75: Which one of the following dienes is
Q111: What is the IUPAC name for the