Which One of the Following Represents the Highest Energy -Antibonding Molecular Orbital of 1,3-Butadiene?
A) I
B) II
Which one of the following represents the highest energy -antibonding molecular orbital of 1,3-butadiene? 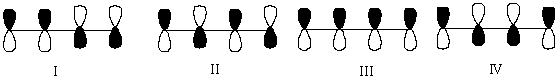
Definitions:
Mutual Interdependence
A condition in which the actions or decisions of each firm in a market affect and are affected by those of other firms, particularly relevant in oligopolistic markets.
Localized Market
A market confined to a specific geographic area, where the products or services offered are tailored to the preferences of the local population.
Profits-Payoff Table
A tabular representation that illustrates the potential profits or payoffs from various business strategies or decisions under different scenarios.
Duopoly
A market structure characterized by two companies controlling the majority of the market share for a good or service.
Q3: Provide the IUPAC name for the following
Q6: What is the IUPAC name for the
Q9: Which of the following is not true
Q13: For the transformation shown, select the most
Q28: Classify the following compound as aromatic, antiaromatic,
Q36: Which diene and dienophile would react to
Q49: Which of the following statements is consistent
Q62: For the transformation shown, select the most
Q101: Concentrated alcohols show a _absorption in the
Q102: Provide the reagent(s) necessary to carry out