The free radical polymerization of styrene with benzoyl peroxide yields a polymer that has repeat units arranged primarily in a 'head-to-tail' arrangement. This means that the phenyl group primarily ends up placed at alternating carbon atoms along the chain. Use correct arrow formalism to show why this arrangement is preferred over a 'head-to-head' or 'tail-to-tail' arrangement. 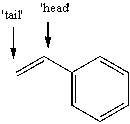
Definitions:
First Year
Refers to the initial period or the first 12 months of a specific timeframe, often used in the context of financial or operational performance.
Absorption Costing
A method of product costing that includes all manufacturing costs, both fixed and variable, in the cost of a product.
Contribution Margin
The amount remaining after variable costs have been subtracted from revenue, which contributes to covering fixed costs and generating profit.
South Business Segment
A term possibly referring to a division or part of a company that operates in the southern region, focusing on geographical business segmentation.
Q4: For the transformation shown, select the most
Q15: Complete reduction of 1 mole of (3E,5Z)-3-methylhepta-3,5-dien-1-yne
Q23: Using Grignard reaction, show how you could
Q49: What is the IUPAC name for the
Q51: Using Grignard reaction, show how you could
Q67: Provide a molecular formula that is consistent
Q83: Provide the IUPAC name for the alkyne
Q101: Provide a stepwise synthesis for the following.
Q122: Which one of the following compounds is
Q128: For the reaction shown, which of the