The lone pair on nitrogen in the following compound is _______. 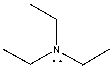
Definitions:
C-H Bonds
Chemical bonds between carbon (C) and hydrogen (H) atoms, forming the backbone structure of organic molecules.
Electronegativity Difference
The difference in electronegativity values between two atoms, determining the polarity of the bond they form.
H-Se Bonds
Refers to the chemical bonds between hydrogen and selenium atoms, commonly found in hydrogen selenide.
Molecular Polarity
A measure of how evenly electric charges are distributed in a molecule, affecting its interactions with other molecules.
Q54: Which of the following choices is a
Q65: What is the % ee of a
Q72: Identify the acid and the base and
Q88: Describe how soaps function as cleaning agents.
Q100: Which of the following is the most
Q104: The bonding pattern of oxygen with a
Q111: Draw in all of the axial hydrogens
Q123: Draw the conjugate acid of the following
Q144: What is the correct order of hybridization
Q186: Which of the indicated carbon atoms have