Predict the enthalpy (ΔH) value for the theoretical reaction below, and indicate whether it is endothermic or exothermic. The bond dissociation energy for each bond in Kcal/mol is shown below each reactant and product. 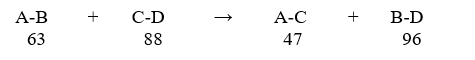
Definitions:
Education
The process of receiving or giving systematic instruction, especially at a school or university.
Protective Factor
Variable that is thought to reduce the poor outcomes associated with adverse circumstances.
Vitamin D
A fat-soluble vitamin essential for the absorption of calcium, support of bone health, and immune function.
Osteoporosis
A condition characterized by weakened bones, making them fragile and more likely to break.
Q23: What is the IUPAC name of the
Q48: Does the alkene shown below violate Bredt's
Q55: What is a micelle?
Q77: Given the bond dissociation energies below (in
Q77: Using solely a fatty acid as your
Q93: Although F is more electronegative than Cl,
Q98: What is the name of the major
Q101: Consider the reaction of A being converted
Q102: A bicyclic alkane contains 12 carbon atoms.
Q120: If a mixture contains 75% of one