Predict the enthalpy (ΔH) value for the theoretical reaction below, and indicate whether it is endothermic or exothermic. The bond dissociation energy for each bond in Kcal/mol is shown below each reactant and product. 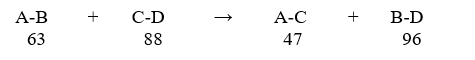
Definitions:
Empowerment
The process of giving power, authority, and confidence to employees, enabling them to take initiative and make decisions.
Stakeholders
Individuals or groups that have an interest in any decision or activity of an organization, including employees, customers, suppliers, and shareholders.
Community Involvement
Engagement of a company or its employees in activities and projects that benefit the society or environment in their local community.
Voluntary Labor
Work undertaken by choice or without financial compensation; often in the context of volunteerism or unpaid internships.
Q7: Which of the statements below accurately describe(s)
Q20: Provide an acceptable name for (CH<sub>3</sub>)<sub>2</sub>CHCH=C(CH<sub>3</sub>)CH<sub>2</sub>CH<sub>3</sub>.
Q21: Cross-linking typically _ the T<sub>m</sub> of a
Q36: _ is the minimum kinetic energy reacting
Q57: What cyclic products results when 1,8-nonadiene is
Q93: Circle all of the structural features of
Q107: Draw an acceptable structure for 4-tert-butyloctane.
Q113: Which of the following statements best describes
Q117: Describe the sources of angle strain and
Q118: Lecithins are phospholipids wherein the alcohol choline