Consider the conversion of X to Z through the sole intermediate Y. Given the reaction-energy diagram shown below, which step is the rate-limiting step? Explain your reasoning. 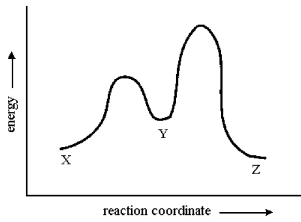
Definitions:
Lewis Structure
A representation that illustrates how atoms in a molecule bond and highlights any unshared electron pairs that might be present.
Lewis Structure
A visual diagram that illustrates the bonding between atoms and the lone pairs of electrons in a molecule.
Phosphine
A colorless, flammable, and toxic gas with the chemical formula PH3, used in various industrial applications and as a pesticide.
Ammonia
A compound of nitrogen and hydrogen with the formula NH3, a colorless gas with a characteristic pungent smell.
Q7: β-Ocimene is a natural product with a
Q11: When 2,2-dimethylbutane is subjected to free-radical chlorination,
Q29: The twisted boat conformation of cyclohexane is
Q31: Terpenes which contain two isoprene units are
Q36: Dehydration of 1-butanol with concentrated sulfuric acid
Q41: How do alkyl substituents stabilize a carbocationic
Q57: How many asymmetric carbons are present in
Q60: When Br radical reacts with 1-butene (CH<sub>3</sub>CH<sub>2</sub>CH=CH<sub>2</sub>),
Q95: Which of the following difluorocyclohexane isomers has
Q102: When isobutylene [CH<sub>2</sub>-C(CH<sub>3</sub>)<sub>2</sub>] is treated with BF<sub>3</sub>,