If the following electrochemical cell is constructed,what voltage will be measured on the voltmeter? 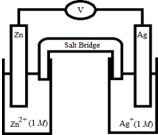 Half-Reaction E° (V) Zn2+(aq) + 2e- →Zn(s) -0.76
Half-Reaction E° (V) Zn2+(aq) + 2e- →Zn(s) -0.76
Ag+(aq) + e- → Ag(s) +0.80
Definitions:
SQL
A standardized language for managing and manipulating relational databases.
Database Content
The data and information stored within a database, including records, fields, and database-specific configurations.
Group Programming
A method in software development where multiple programmers work together on a single project or module.
Proposal
A formal suggestion or plan presented for consideration or discussion by others.
Q5: As the molar mass of a compound
Q43: Amphoteric oxides exhibit both acidic and basic
Q43: A beta particle is a proton.
Q50: Ethylenediaminetetraacetic acid (EDTA)is an effective antidote for
Q70: Find the pH of a 0.183 M
Q80: Which one of the following is not
Q90: What is the meaning of an asterisk
Q95: An equimolar mixture (50:50 ratio)of two enantiomers
Q105: Which of the following is not a
Q116: The maximum oxidation state of an element