Based on the following electrochemical cell,what is the standard reduction potential of metal M at 298 K? (R = 8.314 J/K • mol,F = 96500 C/mol) 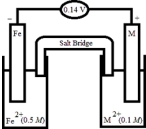 Half-Reaction E° (V) Fe2+(aq) + 2e- → Fe(s) -0.44
Half-Reaction E° (V) Fe2+(aq) + 2e- → Fe(s) -0.44
Definitions:
Cognitive Dissonance
A psychological phenomenon where an individual experiences discomfort due to holding conflicting beliefs, ideas, or values.
Opportunities for Promotion
refer to the prospects or avenues available within an organization for employees to advance to higher positions.
Job Satisfaction
The level of contentment employees feel about their work, which can influence their performance and retention.
Cognitive Dissonance
A psychological phenomenon where an individual experiences discomfort or stress from holding two or more conflicting beliefs, values, or attitudes.
Q2: For stable atoms of elements having low
Q3: What is E°<sub>cell</sub> for the following reaction?
Q4: Which pair of substances is capable of
Q13: What are the two types of stereoisomers
Q25: What is the Henderson-Hasselbach equation?<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6485/.jpg"
Q28: What is the systematic name for Cr(CO)<sub>3</sub>(NH<sub>3</sub>)<sub>3</sub>?<br>A)chromiumtriaminotricarbonyl<br>B)triamminechromium
Q46: What describes the trapping of heat near
Q54: The complete combustion of liquid benzene is
Q102: How should the color of the solution
Q111: The following energy-level diagram could correspond to