Based on the following electrochemical cell,which statement is true? 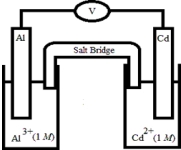 Half-Reaction E° (V) Al3+(aq) + 3e- → Al(s) -1.66
Half-Reaction E° (V) Al3+(aq) + 3e- → Al(s) -1.66
Cd2+(aq) + 2e- → Cd(s) -0.40
Definitions:
Myths
Traditional stories, often with a historical basis, that serve to explain a practice, belief, or natural phenomenon.
Misconceptions
Incorrect or mistaken beliefs or ideas stemming from misunderstanding or lack of knowledge.
Provocatively
In a manner intended to arouse or elicit a strong reaction or interest, often by challenging conventional norms.
Post-traumatic Stress Disorder
A mental health condition triggered by experiencing or witnessing a terrifying event, characterized by severe anxiety, flashbacks, and uncontrollable thoughts.
Q8: What is used to measure the rotation
Q30: Calcium-39 undergoes positron decay.Each positron carries 5.49
Q42: At equilibrium E° = 0.
Q52: In the mid-1980's which of the following
Q67: Which is the weakest acid?<br>A)HBrO<sub>4</sub><br>B)HBr<br>C)HBrO<sub>2</sub><br>D)HBrO<br>E)HBrO<sub>3</sub>
Q82: Consider the dissolution of MnS in water.[K<sub>sp</sub>(MnS)=3.0
Q84: Consider the following balanced redox reaction. Mn<sup>2+</sup>(aq)+
Q93: Beta particles are identical to<br>A)protons.<br>B)helium atoms.<br>C)hydrogen atoms.<br>D)helium
Q122: If Q<K then less reactants are observed
Q131: HCN is classified as a weak acid