If the following electrochemical cell is constructed,what voltage will be measured on the voltmeter? 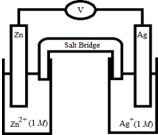 Half-Reaction E° (V) Zn2+(aq) + 2e- →Zn(s) -0.76
Half-Reaction E° (V) Zn2+(aq) + 2e- →Zn(s) -0.76
Ag+(aq) + e- → Ag(s) +0.80
Definitions:
Ending Inventory Balance
The value of all the unsold goods that a company has in its possession at the end of an accounting period.
Goods In Transit
Items that have been shipped by a seller but have not yet been received by the purchaser, often included in accounting considerations for inventory management.
Inventory Valuation
The method used to assign a monetary value to inventory, including FIFO, LIFO, and weighted average cost methods.
Current Cost
The cost that would be incurred to replace an asset or to purchase a service at the present time as opposed to its historical cost.
Q13: The equilibrium constants (expressed in atm)for the
Q22: Which isotope,when bombarded with bismuth-209,would yield two
Q43: Amphoteric oxides exhibit both acidic and basic
Q76: Suppose 0.015 mol of KOH is added
Q81: Solids containing donor impurities are called _
Q97: _ is the way to identify the
Q106: How many 3d electrons does a ground-state
Q113: At 450°C,tert-butyl alcohol decomposes into water and
Q116: At equilibrium,the rate of the forward reaction
Q119: As a result of beta decay,the product