Below are energy level diagrams for two different atoms. 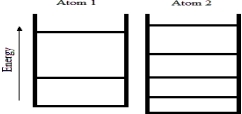 Based on the diagrams,which atom has the larger standard molar entropy at T = 298 K?
Based on the diagrams,which atom has the larger standard molar entropy at T = 298 K?
Definitions:
Bailee
A person or entity that has been entrusted with temporary possession, but not ownership, of personal property by another, known as the bailor.
Commercial Aircraft
Airplanes designated for carrying passengers or goods, operated by airlines or cargo carriers, as opposed to military or private aircraft.
Flight Attendant
An airline employee responsible for ensuring the safety, comfort, and service to passengers aboard flights.
Bailment for Reward
A legal relationship that involves the temporary transfer of personal property to another for a specified fee or reward.
Q10: For the reaction SO<sub>2</sub>(g)+ NO<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6485/.jpg"
Q24: The Earth's core consists mainly of<br>A)Ni.<br>B)O.<br>C)Al.<br>D)Si.<br>E)Fe.
Q41: For the reaction PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6485/.jpg"
Q78: What is the name for a solute
Q83: Volcanoes<br>A)produce about two-thirds of the sulfur in
Q85: When the concentrations of both the reactants
Q109: The process in which a gaseous substance
Q112: What happens to the solution if sodium
Q114: What is the name that refers to
Q130: What colors do excited oxygen atoms emit?