Suppose 50.0 mL of a 0.100 M solution of the weak acid HA is titrated with a 0.100 M solution of NaOH.Which diagram best represents the equilibrium composition of the solution at point X on the titration curve below? (Solvent water molecules are omitted for clarity.) 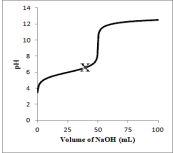
Definitions:
Electrolyte Imbalance
A condition where the levels of electrolytes (minerals such as sodium, potassium, and calcium) in the body are either too high or too low, affecting bodily functions.
Tertiary Prevention
Interventions designed to manage and alleviate the symptoms of an already diagnosed disease or to prevent disease progression and complications.
Primary Prevention
Measures taken to prevent the onset of a disease or injury before it occurs, focusing on reducing risk factors.
Tertiary Prevention
Measures taken to manage and contain disease progression after it has established to prevent further complications or deterioration.
Q14: A voltaic cell consists of an Au/Au<sup>3+</sup>
Q15: If one starts with pure NO<sub>2</sub>(g)at a
Q26: What is the nuclear binding energy per
Q34: When the following redox equation is balanced
Q35: When radon decays it produces Po-214 and
Q57: Which of the following has ΔG°<sub>f</sub> =
Q71: Which of the following aqueous solutions should
Q76: _ _ is the percentage of dissolved
Q117: A solution is prepared by adding 0.10
Q134: What is the percent CdSO<sub>4</sub> by mass