Below is a representation of the sparingly soluble salt MX at equilibrium in aqueous solution.(Each circle represents 1.0 ×10-3 mol of atoms,and the volume of the box is 1.0 L.Solvent water molecules are omitted for clarity.) 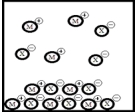 What is Ksp for MX?
What is Ksp for MX?
Definitions:
Q17: The solubility of lead(II)chloride is 0.45 g/100
Q17: Which statement is correct?<br>A)If K < 1,lnK
Q19: What is the conjugate base of water?<br>A)H<sub>3</sub>O<sup>+</sup><br>B)OH<sup>-</sup><br>C)H<sub>3</sub>O<br>D)OH<br>E)H<sub>2</sub>O<sub>2</sub>
Q26: For PbCl<sub>2</sub> (K<sub>sp</sub> = 2.4 × 10<sup>-4</sup>),will
Q33: Which of the following will decrease the
Q52: How does the entropy change when a
Q57: Which is an acidic oxide?<br>A)P<sub>4</sub>O<sub>10</sub><br>B)MgO<br>C)Fe<sub>2</sub>O<sub>3</sub><br>D)K<sub>2</sub>O<br>E)Cr<sub>2</sub>O<sub>3</sub>
Q75: Substitutes for CFCs<br>A)contain C-H bonds that make
Q109: Which is the strongest acid?<br>A)SO<sub>4</sub><sup>2-</sup><br>B)H<sub>2</sub>SO<sub>3</sub><br>C)H<sub>2</sub>SO<sub>4</sub><br>D)HSO<sub>4</sub><sup>-</sup><br>E)HSO<sub>3</sub><sup>-</sup>
Q112: Which is the correct equilibrium constant expression