Below is a representation of an aqueous solution of a weak acid HA at equilibrium.(Each circle represents 1.0 mmol of atoms,and the volume of the box is 1.0 L.Solvent water molecules are not shown for clarity.) 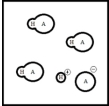 What is the percent ionization of HA?
What is the percent ionization of HA?
Definitions:
Operating Expenses
Costs associated with the day-to-day operations of a business, excluding cost of goods sold (COGS).
Tax Liability
The total amount of tax that an individual or corporation is legally obligated to pay to an authority as the result of the occurrence of a taxable event.
Dividends Paid
Cash or other forms of payouts made by a company to its shareholders from its earnings.
Net Cash Flow
The difference between a company's cash inflows and outflows in a given period.
Q50: What is E°<sub>cell</sub> for a galvanic cell
Q52: At elevated temperatures,hydrogen iodide may decompose to
Q54: What would you observe if you set
Q56: A 0.15 M solution of chloroacetic acid
Q66: Below is a representation of a sealed
Q70: When a<sup>87</sup> Br nucleus emits a beta
Q90: Based on the data presented below,which is
Q94: What is the cell potential at 25°C
Q104: When a strong acid is titrated with
Q107: Which has the highest surface tension at