Below is a representation of an aqueous solution of a weak acid HA at equilibrium.(Each circle represents 1.0 ×10-3 mol of atoms,and the volume of the box is 1.0 L.Solvent water molecules are not shown for clarity.) 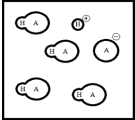 What is Ka of HA?
What is Ka of HA?
Definitions:
Staffing Table
A Staffing Table is a document or tool used in human resources planning that outlines the current and future staffing needs of an organization, including positions and number of employees required.
HR Demand Requirement
The specific human resource needs of an organization, based on its strategic planning and operational requirements.
Level And Function
Terms used to categorize the hierarchy and job role within an organization, respectively, determining the responsibility, work complexity, and possibly the pay grade.
Quantitative And Qualitative Methods
Research methods involving the collection and analysis of numerical data for statistical analysis and the examination of non-numerical data to understand concepts, thoughts, or experiences, respectively.
Q4: Nuclei with an _ number of protons
Q9: Liquid crystals are anisotropic,because the properties they
Q15: You have 500.0 mL of a buffer
Q32: A solution is prepared by adding 0.10
Q71: What name is given to a polymer
Q95: Which diagram best represents the products when
Q96: The substance HClO<sub>4</sub> is considered to be<br>A)a
Q102: _ is the value for i,the van't
Q110: In a nuclear reaction elements are converted
Q120: Formic acid,which is a component of some