For the endothermic reaction A2(g)  2A(g) ,a snapshot of an equilibrium mixture of A(g) and A2(g) at low temperature may look as follows.(Each circle represents 1.0 mol of A atoms,and the volume of the box is 1.0 L.)
2A(g) ,a snapshot of an equilibrium mixture of A(g) and A2(g) at low temperature may look as follows.(Each circle represents 1.0 mol of A atoms,and the volume of the box is 1.0 L.) 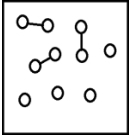 If the system pressure is lowered,what might the new equilibrium system look like?
If the system pressure is lowered,what might the new equilibrium system look like?
Definitions:
Scientific Management
A theory of management that analyzes workflows to improve economic efficiency and labor productivity.
Hourly Wages
Compensation paid to employees based on the number of hours worked, typically expressed as an amount of money per hour.
Divided Tasks
The breaking down of tasks into smaller, more manageable parts to be completed by different individuals or teams, often to increase efficiency.
Piece Rates
A pay system where employees are paid based on the quantity of units they produce or tasks they complete.
Q4: What type of polymer is represented by
Q9: At temperatures below 273 K,it is observed
Q9: a.Name the two unit cells which occur
Q26: What is the name of the C<sub>60</sub>
Q44: Polymers where all the substituents are in
Q46: The polymer formed from the monomer CH<sub>2</sub>=CH-CN
Q68: Sodium carbonate,Na<sub>2</sub>CO<sub>3</sub>(s),may be prepared by heating sodium
Q90: Which is defined as the maximum amount
Q104: What is defined as a fraction with
Q139: Cadmium bromide is used in photography and