Below is a diagram representing a solvent A(l) in a 1-L beaker,and a solute X dissolved in the solvent.Solvent A has a density of 0.8 g/mL,and a molar mass of 40 g/mol. Solute X has a molar mass of 30 g/mol.Each circle of X represents 1 mol of X.Assume that the solute addition does not significantly change the volume of liquid in the beaker. 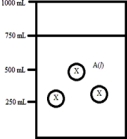 What is the mass percent of solute X in this solution?
What is the mass percent of solute X in this solution?
Definitions:
Investment Bankers
Professionals who specialize in raising capital for companies, governments, and other entities by underwriting or acting as the agent in the issuance of securities.
Initial Public Offerings (IPOs)
The process by which a private company goes public by selling its shares to the general public for the first time.
Electronic Communication Network (ECN)
A type of computer system that facilitates trading of financial products, such as stocks and currencies, outside traditional stock exchanges, allowing direct trading between parties.
Stock IPO
An Initial Public Offering, the process by which a private company goes public by offering its stock for the first time to the general public and other investing institutions.
Q5: A sample of nitrogen gas has the
Q13: Liquids are anisotropic,because their properties are independent
Q25: The reaction of nitrogen with oxygen to
Q30: The segment represents the polymer named <img
Q31: _ _ is another name given for
Q35: What is the [H<sub>3</sub>O<sup>+</sup>] in a buffer
Q73: For the equilibrium A<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6485/.jpg" alt="For
Q80: Describe the materials used in joint replacements.
Q100: Suppose 50.0 g of N<sub>2</sub>O<sub>4</sub> is introduced
Q110: Which statement is true regarding the reversible