Below is a diagram representing a solvent A(l) in a 1-L beaker,and a solute X dissolved in the solvent.Solvent A has a density of 0.8 g/mL,and a molar mass of 40 g/mol. Solute X has a molar mass of 30 g/mol.Each circle of X represents 1 mol of X.Assume that the solute addition does not significantly change the volume of liquid in the beaker. 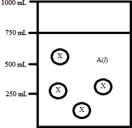 What is the mole fraction of the solute X in this solution?
What is the mole fraction of the solute X in this solution?
Definitions:
Wage Rate
The amount of money paid to an employee per unit of time or per task performed, often expressed as an hourly rate.
Budget Equation
A mathematical representation summarizing the balance between an individual's or organization's income and expenditures.
Leisure
Free time available to a person away from duties, work, or other compulsory activities.
Nonlabor Income
Income received that does not originate from employment or work efforts, such as dividends, interest, or rental income.
Q3: According to the VSEPR model,a molecule with
Q10: The zincblende structure of ZnS has the
Q74: Increasing the temperature of an exothermic reaction
Q75: Which is not a property of a
Q82: Consider the reversible reaction: 2NO<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6485/.jpg"
Q102: Find the pH of a 0.135 M
Q107: What are the units of the constant
Q113: Aqueous ammonia is commercially available in a
Q126: For a homonuclear diatomic molecule,which molecular orbital
Q128: Select the strongest electrolyte from the following