Below is a diagram representing a solvent A(l) in a 1-L beaker,and a solute X dissolved in the solvent.Solvent A has a density of 0.8 g/mL,and a molar mass of 40 g/mol. Solute X has a molar mass of 30 g/mol.Each circle of X represents 1 mol of X.Assume that the solute addition does not significantly change the volume of liquid in the beaker. 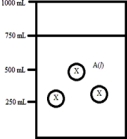 What is the mass percent of solute X in this solution?
What is the mass percent of solute X in this solution?
Definitions:
Residual Stage
A phase in some diseases, after the main symptoms have disappeared, where a few mild symptoms may still persist.
Alogia
A reduction in the amount of speech and/or speech content, often observed in schizophrenia and other psychotic disorders.
Avolition
A lack of motivation or inability to initiate and persist in goal-directed activities, often observed as a symptom in some psychiatric disorders such as schizophrenia.
Delusions of Grandeur
A false belief in one's own superiority, greatness, or power, often found in psychiatric conditions such as bipolar disorder and schizophrenia.
Q35: Which is the correct mass-action expression,Qc,for the
Q58: If 2.38 mol of a gas has
Q87: The temperature of the carbon dioxide atmosphere
Q93: Nitric oxide and bromine were allowed to
Q95: Does a precipitate of magnesium fluoride form
Q103: The stronger the acid,the weaker its conjugate
Q109: Which is the strongest acid?<br>A)SO<sub>4</sub><sup>2-</sup><br>B)H<sub>2</sub>SO<sub>3</sub><br>C)H<sub>2</sub>SO<sub>4</sub><br>D)HSO<sub>4</sub><sup>-</sup><br>E)HSO<sub>3</sub><sup>-</sup>
Q111: Consider the reaction N<sub>2</sub>(g)+ O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6485/.jpg"
Q117: The solubility of magnesium phosphate is 2.27
Q117: A solution is prepared by adding 0.10